CHEMISTRY CHAPTER ZERO
Cautions
I encourage each teacher who considers using the approach and/or materials on this website to consider the following cautions:
CAUTION #1: Using the Chemistry Chapter Zero approach may appear to your colleagues as "Not teaching the chemistry they will need for ..."
There is a well-established sequence of chemistry courses in the United States for earning a bachelor's degree in chemistry. In the freshman year, one takes a year of General Chemistry with lab. In the sophomore year, one takes a year of Organic Chemistry with lab, and perhaps, a one semester course in Quantiative Analysis. In the junior year, one takes a year of Physical Chemistry and perhaps a one semester course in Instrumental Analysis. In this sequence, instructors of courses after the freshman year have a very good idea of what was covered in the General Chemistry course, and they presume (rightly or wrongly!) that the students in their courses can build on that freshman year material.
The bachelor's degree sequence strongly influences the high school curriculum. Teachers of high school chemistry classes often get feedback as their students return home for Christmas break, and as good teachers, they adapt the high chemistry course they teach to provide better preparation for the college General Chemistry course. The correspondence between high school AP chemistry courses and the college General Chemistry course is even stronger -- the AP course is often accepted in lieu of General Chemistry.
The influence extends further down into lower grades. Many "introductions to chemistry" are a first pass at the curriculum in the high school chemistry course.
The Chemistry Chapter Zero approach argues that students who actually can work with the concepts of atoms (and a periodic table), reactions, and properties are (at least as) capable of moving forward when the material is presented in the symbol-rich standard presentation.
CAUTION#2: The students may not perform well on standardized tests, which are, of course, developed to measure student performance in the standard courses.
Consider the following test question, taken from the April 2006 version of the Texas TAKS test for 8th graders. http://www.tea.state.tx.us/student.assessment/resources/release/taks/index.html
Which letter in this model of a boron atom represents a neutron?”
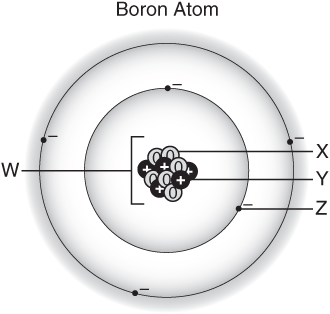
This is a fine test question; it requires reasoning rather than simple memorization. However the approach in Chemistry Chapter Zero focuses on atoms as the "indivisible particles" rather than on presenting information about the internal structure of atoms. It is possible that students could have acquire a substantial and important foundation for future learning in the standard chemistry course and NOT be able to answer this TAKS test question.
Enough, enough!
Show me how you can help me learn chemistry!